|
|
Genetics Problem Spaces
|
|
Behavioral Genetics Intro
Exercise Student Projects |
Behavioral
Genetics Introductory Exercise Introduction
The nematode Caenorhabditis has been successfully used to dissect the genetic basis of an array of complex biological phenomena. Recently a Nobel Prize in Medicine was awarded for studies with C. elegans on the genetic control of animal development. Another hot area of current research with C. elegans is animal behavior. These nematodes exhibit a wide array of behaviors that can be monitored in the laboratory. One well characterized behavior is chemotaxis. Basically, chemotaxis is movement toward or away from a chemical. More specifically, it involves a change in direction of movement in response to a gradient of a chemical. Positive chemotaxis is movement towards higher concentrations of the chemical; negative chemotaxis is movement towards lower concentrations of the chemical. Several chemicals have been identified that elicit a positive or negative chemotaxis response in C. elegans. To identify the genes involved in chemotaxis, several mutant strains of C. elegans have been generated that are defective for this behavior. Typically these mutant nematodes fail to change direction of movement in response to the chemical gradient. Some of the mutant strains are highly specific to one type of chemical. Other mutants are defective in response to a wide range of chemicals. These genes have been found to encode a wide variety of proteins, including olfactory receptors, transcription factors and neuron secretory proteins. A compound that elicits an unusual and complex chemotaxis response is benzaldehyde. In a gradient of high concentrations of benzaldehyde, C. elegans initially shows a positive chemotaxis response and moves in the direction of higher concentrations. This positive response lasts approximately 20 – 30 minutes. At that point, the behavior of the nematode reverses and it shows a negative chemotaxis response and moves in the direction of lower concentrations. This change in behavior is often cited as an example of simple learning in C. elegans. In this laboratory exercise, you will use chemotaxis mutants to “genetically dissect” the complex behavior elicited by benzaldehyde. Specifically you will be investigating whether the same genes required for the positive chemotaxic response to benzaldehyde are required for the negative response to benzaldehyde. Specific Goals
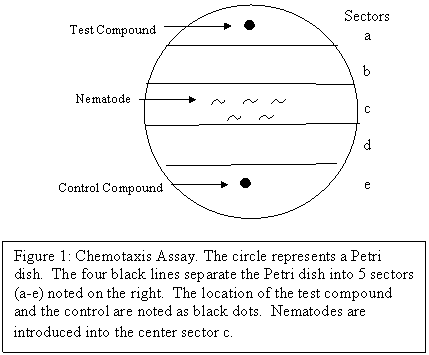
Chemotaxis Assay Several simple assays have been used to measure chemotaxis responses. In this exercise we will assay chemotaxis in a Petri dish. A simple test arena is setup in agar Petri plate (Figure 1). Evenly spaced lines drawn on the bottom of the plate are used to divide the plate into 5 sectors (a-e). At one end of the plate (sector a) a small amount of the compound to be tested is spotted. At the opposite end (sector e), a control compound may be spotted (usually water). Nematodes are placed in the center of the plate (sector c) and allowed to move around for one hour. If they have a positive chemotaxis response to the test compound they will move from the center of the plate towards the test compound. If they have a negative chemotaxis response to a compound they will move away from the test spot and end up near the water spot. To observe the dynamic behavior of the nematodes in response to benzaldehyde, they must be monitored every 10 minutes. To quantify the kinetics of movement in response to a compound the number of worms in each sector is determined. A chemotaxis index (WCI) is calculated. [2(# in a) + (# in b)] – [2(# in e) + (# in d)]
(total # of nematodes on the plate)
Exercise
Lab Report
Hypothesis I: The positive and the negative chemotaxic response to benzaldehyde used the same genetic pathway (require the same gene products). Hypothesis II: The positive and the negative chemotaxic response to benzaldehyde are different genetic pathways and each requires unique gene products.
Write a paragraph analyzing your data with regard to these two hypotheses. |